Advertisements
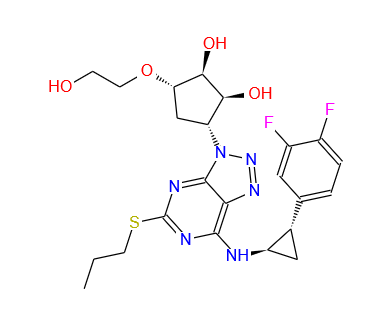

TICAGRELOR
| Price: | US$ 1600 / Kilogram |
|---|---|
| Minimum Order: | |
| Payment Terms: | T/T IN ADVANCE |
| Port of Export: |
Product Details
| Model No.: | Brand Name: |
|---|
| Certification: | |
|---|---|
| Specification: |
Molecular Formula: C23H28F2N6O4S
Formula Weight: 522.57 CAS No.: 274693-27-5 |
Packaging & Delivery
| Packaging: | |
|---|---|
| Delivery/Lead Time: | |
| Production Capacity: |
Product Description
Indications and Usage Ticagrelor, a new platelet aggregation inhibitor successfully developed by AstraZeneca (U.S.), is the world’s first reversible combination orally-administered P2Y12 adenosine diphosphate receptor antagonist.
It is used to reduce cardiovascular death and heart attacks in patients with acute coronary syndrome (ACS.) Rapid onset after oral administration, and can effectively improve symptoms of patients with ACS. Thienopyridines is a reversible P2Y12 inhibitor, so it is particularly applicable towards patients who need to undergo anticoagulant therapy before surgery.
Mechanisms of Action Reversibly acts on the 2 purine receptor (purinoceptor 2, P2,) subtype P2Y12, of vascular smooth muscle cells (VSMC,) does not require metabolic activation, and has a significant inhibitory effect on platelet aggregation induced by adenosine diphosphate (ADP.)
Clinical Research Ticagrelor was approved by the U.S. Food and Drug Administration (FDA) on the basis of PLATO clinical studies. PLATO was a 43 country, 862 center randomized, double-blind, multi-centered study which included 18,624 patients hospitalized for ACS. Patients were randomly given either aspirin plus ticagrelor, or aspirin plus clopidogrel, two antiplatelet regimens. The study showed a 16% reduction in risk of heart attack, stroke, or death in the group taking Ticagrelor compared to those who took clopidogrel. The PLATO study demonstrated the benefits of Ticagrelor for patient survival, and is currently widely recommended as a treatment strategy for ACS. In addition, at the same time it reduces operative myocardial infarction and thrombosis after stent implantation, it also does not increase the overall risk of fatal bleeding and other serious adverse effects.
Ticagrelor however has two shortcomings. First, it must be taken twice a day, compared to once a day for clopidogrel, and secondly, it may cause adverse effects such as breathing difficulties, significantly more than with the latter. Its half-life is only 12 hours, and taken twice a day, a challenge for patients with poor compliance. In practice, it has also been found that around 20% of patients treated with clopidogrel do not follow their prescriptions, and these patients are even less likely to take the prescribed doses when using ticagrelor. Thus, as a rapidly reversible drug, direct withdrawal will likely increase the risk of acute thrombosis, causing myocardial infection or stroke.
Description In December 2010, the P2Y12 receptor antagonist ticagrelor (also known as AZD6140) was approved in Europe for the treatment of acute coronary syndrome (ACS), a condition that covers several clinical symptoms with the potential to cause acute myocardial ischemia (MI). ADP binds to two purinergic receptors, the P2Y1 and P2Y12 receptors. The action of ADP binding to the P2Y12 receptor results in activation of the GP Ⅱb/Ⅲa (integrin) receptor.GP Ⅱb/Ⅲa initiates and prolongs platelet aggregation, which in turn results in the cross-linking of platelets through fibrin and finally thrombus formation. Inhibition of ADP stimulation of the P2Y12 receptor has been found to be an effective strategy for managing the atherothrombotic events associated with ACS and potentially resulting from percutaneous coronary intervention (PCI, stent implantation) .

|
SUPPLIER PROFILE
|
|||
|---|---|---|---|
| Company: | HangZhou HuiSheng Biotech Pharmaceutical Co., Ltd | ||
| City/State | Hangzhou, Zhejiang | Country: |
China 
|
| Business Type: | Export - Manufacturer / Trading Company | Established: | NA |
| Member Since: | 2017 | Contact Person | Michael Liew |
SUPPLIER PROFILE
City/State/Country -
Hangzhou, Zhejiang
China 

Business Type -
Export - Manufacturer / Trading Company
Established -
NA
Member Since -
2017
Contact Person -
Michael Liew



