Advertisements


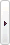
Shine i1910 Fully Automatic Chemiluminescence Immunoassay Analyzer
| Price: | US$ 1 / Barrel |
|---|---|
| Minimum Order: | |
| Payment Terms: | TT |
| Port of Export: |
Product Details
| Model No.: | Brand Name: |
|---|
| Certification: | |
|---|---|
| Specification: | Shine i1910 Fully Automatic Chemiluminescence Immunoassay Analyzer |
Packaging & Delivery
| Packaging: | |
|---|---|
| Delivery/Lead Time: | |
| Production Capacity: |
Product Description
Shine i1910 is a fully automatic and simple-to-use benchtop chemiluminescence immunoassay analyzer with a throughput of 120 tests per hour. Based on various modes of antigen-antibody immunoreactivity and with paramagnetic particles as a carrier, i1910 utilizes the indirect chemiluminescence reaction of AMPPD with alkaline phosphatase to realize catalytic cracking of AMPPD under the action of enzymes. As a fully automated immunoassay analyzer with high quality, Shine i1910 with the characteristics of simplicity, reliability and flexibility, which can help optimize your laboratory analysis performance.
Automated clia analyzer Features
◆ Innovative design: Incubation, washing and detection all-in-one.
◆ Fast: first result in 15 mins.
◆ Good repeatability: Batch CV≤8%.
Automatic Chemiluminescence Immunoassay Analyzer Specifications
Sample Type Human serum and plasma samples
Throughput Test speed up to 120 tests / hour
Assay cup Disposable assay cup
Sample position 30
Reagent position 10
Reagent refrigeration 24h refrigeration, reagent disk temperature: 2 ®C ~8 ®C
Temperature stability of reaction disk 37 ℃¯ 0.3 ℃, and the temperature fluctuation should not exceed 0.2℃
Repeatability within batch CV ≤ 8%
Carry-over ≤10-5
Linear correlation r ≥ 0.99, the concentration range is not less than 2 orders of magnitude
Dimensions (mm) L×W×H 433×679×638
Net weight (kg) 62
Clia Immunoassay Analyzer Test Items
Tumor Markers
Name Abbr.
Carcinoembryonic Antigen Quantitative Diagnostic Kit (CLIA) CEA
Neuron-specific Enolase Quantitative Diagnostic Kit (CLIA) NSE
Carbohydrate Antigen 19-9 Quantitative Diagnostic Kit (CLIA) CA19-9
Carbohydrate Antigen 15-3 Quantitative Diagnostic Kit (CLIA) CA15-3
Carbohydrate Antigen 50 Quantitative Diagnostic Kit (CLIA) CA50
Cytokeratin 19-fragments Quantitative Diagnostic Kit (CLIA) CYFRA21-1
Cancer Antigen 125 Diagnostic Kit (CLIA) CA125
Fertility
Name Abbr.
Estradiol Quantitative Diagnostic Kit (CLIA) E2
Luteinizing Hormone Quantitative Diagnostic Kit (CLIA) LH
Follicle Stimulating Hormone Quantitative Diagnostic Kit (CLIA) FSH
Prolactin Quantitative Diagnostic Kit (CLIA) PRL
Testosterone Quantitative Diagnostic Kit (CLIA) TES
Total β Human Chorionic Gonadotrophin Quantitative Diagnostic Kit (CLIA) Tβ-hCG
Progesterone Quantitative Diagnostic Kit (CLIA) PROG
Placental Growth Factor Diagnostic Kit (CLIA) PLGF
Soluble Fms-like Tyrosine Kinase-1 Diagnostic Kit (CLIA) sFlt-1
Pernatal Screening
Name Abbr.
Alpha-Fetoprotein Quantitative Diagnostic Kit (CLIA) AFP
Alpha-fetoprotein Quantitative Diagnostic Kit (CLIA) Ps-AFP
Pregnancy Associated Plasma Protein A Diagnostic Kit (CLIA) PAPPA
Free β Human Chorionic Gonadotrophin Diagnostic Kit (CLIA) freeβ-HCG
Free Estriol Diagnostic Kit (CLIA) FE3
Infectious Disease
Name Abbr.
2019 Novel Coronavirus (2019-nCoV) Antigen Qualitative Diagnostic Kit (CLIA) 2019-nCoV
2019-nCoV Neutralizing Antibody Detection Kit (CLIA) 2019-nCoV Nab
Anemia
Name Abbr.
Ferritin Quantitative Diagnostic Kit (CLIA) Fer
Kidney Function
Name Abbr.
Neutrophil Gelatinase-Associated Lipocalinn Quantitative Diagnostic Kit (CLIA) NGAL
Thyroid
Name Abbr.
Total Thyroxine (TT4) Quantitative Diagnostic Kit (CLIA) TT4
Free Thyroxine (FT4) Quantitative Diagnostic Kit (CLIA) FT4
Total Triiodothyronine (TT3) Quantitative Diagnostic Kit (CLIA) TT3
Thyroid Stimulating Hormone Quantitative Diagnostic Kit (CLIA) TSH
Free Triiodothyronine (FT3) Quantitative Diagnostic Kit (CLIA) FT3
Thyroglobulin Quantitative Diagnostic Kit (CLIA) TG
Autoimmune
Name Abbr.
Anti-thyroglobulin Antibody Quantitative Diagnostic Kit (CLIA) Anti-TG
Anti-thyroid Peroxidase Antibody Quantitative Diagnostic Kit (CLIA) Anti-TPO
Cardiac
Name Abbr.
D-Dimer Quantitative Diagnostic Kit (CLIA) D-Dimer
Cardiac Fatty Acid Binding Protein Quantitative Diagnostic Kit (CLIA) H-FABP
N-terminal Atrial Natriuretic Peptide Quantitative Diagnostic Kit (CLIA) NT-proBNP
Cardiac Troponin T Quantitative Diagnostic Kit (CLIA) cTnT
Cardiac Troponin I Quantitative Diagnostic Kit (CLIA) cTnI
Myoglobin Quantitative Diagnostic Kit (CLIA) MYO
Creatine Kinase Isoenzyme (CK-MB) Quantitative Diagnostic Kit (CLIA) CK-MB
Chemiluminescence Immunoassay Analyzer Principle
Based on various antigen-antibody immunoreaction modes, the full automatic hemiluminescence immunoassay analyzer takes paramagnetic particles as the carrier, and makes use of the direct chemiluminescence method of acridinium ester. It produces unstable intermediates in excited state under the action of free energy released in chemical reaction. When the intermediates get back to the ground state from the excited state, the photons with the same energy level are released. At this point, it measures such photons and thus conducts the quantitative analysis. The luminescent intensity of the reaction is proportional to the concentration in the sample within the detection range.
There are many chemiluminescence immunoassay analyzer manufacturers, but we are one of the best choices for you.
Want to know the details of molecular diagnostics instruments, contact us.

|
SUPPLIER PROFILE
|
|||
|---|---|---|---|
| Company: | Daan Gene Co., Ltd. | ||
| City/State | gaunzhou, | Country: |
China 
|
| Business Type: | Export - Manufacturer / Trading Company | Established: | NA |
| Member Since: | 2023 | Contact Person | en daangene |
SUPPLIER PROFILE
City/State/Country -
gaunzhou,
China 

Business Type -
Export - Manufacturer / Trading Company
Established -
NA
Member Since -
2023
Contact Person -
en daangene



