Advertisements

Peripheral Stent System
| Price: | US$ 45 / Piece |
|---|---|
| Minimum Order: | 10 |
| Payment Terms: | 100% T/T in advance |
| Port of Export: |
Product Details
| Model No.: | Brand Name: | Zyloxtb |
|---|
| Certification: | |
|---|---|
| Specification: |
OVERVIEW
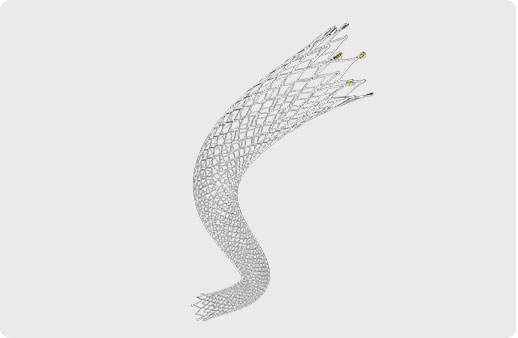
Peripheral Stent System The ZENFLEX¬ peripheral vascular disease stent system is designed to deliver a self-expanding stent to the iliac artery, superficial femoral arteries and/or proximal popliteal arteries via a 6F delivery system. |
Packaging & Delivery
| Packaging: | |
|---|---|
| Delivery/Lead Time: | 2-12 weeks |
| Production Capacity: | 5 |
Product Description
OVERVIEW
Peripheral Stent System
The ZENFLEX¬ peripheral vascular disease stent system is designed to deliver a self-expanding stent to the iliac artery, superficial femoral arteries and/or proximal popliteal arteries via a 6F delivery system.
PRODUCT FEATURES
UNIQUE SPIRAL DESIGN
Excellent flexibility
Superior vascular compliance
EXCELLENT VASCULAR COMPLIANCE
Every 2mm length contains 7 connecting bridges
MICROMESH DESIGN
High radial strength and good fracture resistance
Maximizes vessel patency by decreasing tissue prolapse
GOLD/TANTALUM MARKER
Each end of the stent embedded 6 micro markers
Accurately mark stent ends out and enhances visibility
CONTROLLED RELEASE SYSTEM
One-handed stent deployment
The initial precise positioning followed by well matching quick release
Ergonomic handle with anti-skid tuning dial combines comfortable hand feel and placement accuracy
Compatible with 0.035"guidewire
INDICATIONS
Peripheral Stent System
The ZENFLEX¬ Peripheral Stent System is indicated for improving luminal diameter in the treatment of patients with de novo or restenotic native lesion(s) of the iliac artery, superficial femoral artery and/or proximal popliteal artery with total lesion length up to 150 mm and with a reference vessel diameter ranging from 3 mm to 8 mm.
If you want to know more details of pad stent surgery recovery and leg artery stent surgery, please visit our website.
Zylox-Tonbridge Medical Technology Co., Ltd. ("Zylox-Tonbridge" or "the Company", "2190.HK") announced on February 28 that it has received approval from Hong Kong Stock Exchange, regarding implementation of full circulation of its H shares. The approval was for the conversion and listing of the Company’s 194 million domestic unlisted shares.

|
SUPPLIER PROFILE
|
|||
|---|---|---|---|
| Company: | ZYLOX-TONBRIDGE MEDICAL TECHNOLOGY CO., LTD. | ||
| City/State | Hangzhou, Zhejiang | Country: |
China 
|
| Business Type: | Export - Manufacturer / Trading Company | Established: | 2012 |
| Member Since: | 2022 | Contact Person | Zyloxtb com |
SUPPLIER PROFILE
City/State/Country -
Hangzhou, Zhejiang
China 

Business Type -
Export - Manufacturer / Trading Company
Established -
2012
Member Since -
2022
Contact Person -
Zyloxtb com



