Advertisements
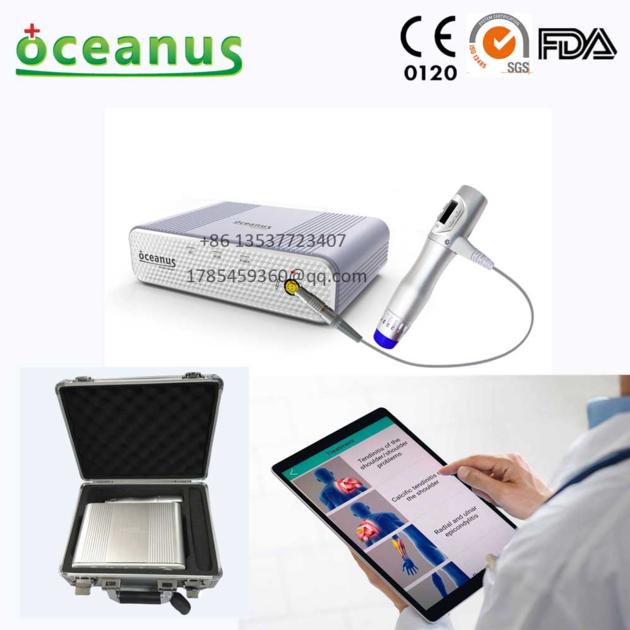

Mobile Shock Wave Therapy device(ESWT) for Physiotherapy/physiotherapy & rehabilitation device
| Price: | US$ 1 / Unit |
|---|---|
| Minimum Order: | 1 |
| Payment Terms: | FOB, CIF, EXW |
| Port of Export: | Shenzhen |
Product Details
| Model No.: | OCE-ESWT-003 | Brand Name: | Oceanus/OEM |
|---|
| Certification: | CE, ISO 13485, FDA |
|---|---|
| Specification: |
Technical specification:
Model Number OCE-ESWT-003 Brand name Oceanus / OEM Instrument classification Class II Supply Ability 100 Unit/Units per Month Display Mode OLED display Energy Level 10 to 185 mJ (equivalent to 0.1~5 bar) Pressure Range 0.1~5 bar Operating Frequency 1~22 Hz Input voltage 100/240V AC Power Consumption 100/240VAC 50/60 Hz,250VA Fuse wire 250V/10A Service life of handle Minimum 4 million shocks Work enviroment requires 20 ºC -32 ºC 25% -75% RH 700hPa-1060hPa Storage environment requires 0 ºC -40 ºC 10% -93% RH 700hPa-1060hPa Transportation environment requires 10 ºC-70 ºC, 10% -93% RH, 700hPa-1060hPa |
Packaging & Delivery
| Packaging: | carton, there is also a aluminum alloy case package inside |
|---|---|
| Delivery/Lead Time: | within 3 days |
| Production Capacity: | 100 Unit/Units per Month |
Product Description
Work principle:
Compressor free ballistic radial shock wave therapy system with electromagnetic generator as projectile accelerator. The hand piece contains a projectile that is accelerated through the electromagnetic transfer of kinetic energy. This kinetic energy is then transformed into impact energy in the applicator head. The impact energy delivered from the applicator head results in radial pulse in the target tissue.
Technical specification:
Model Number OCE-ESWT-003
Brand name Oceanus / OEM
Instrument classification Class II
Supply Ability 100 Unit/Units per Month
Display Mode OLED display
Energy Level 10 to 185 mJ (equivalent to 0.1~5 bar)
Pressure Range 0.1~5 bar
Operating Frequency 1~22 Hz
Input voltage 100/240V AC
Power Consumption 100/240VAC 50/60 Hz,250VA
Fuse wire 250V/10A
Service life of handle Minimum 4 million shocks
Work enviroment requires 20 ºC -32 ºC 25% -75% RH 700hPa-1060hPa
Storage environment requires 0 ºC -40 ºC 10% -93% RH 700hPa-1060hPa
Transportation environment requires 10 ºC-70 ºC, 10% -93% RH, 700hPa-1060hPa
Feature:
*Small volume-1/3 of portable type
*Complete functions-almost the same as the portable type
*Easy to control-no foot switch, operate on the handle directly
*Modern look-the smallest one
*Bluetooth 4.0 communication function-connect with smart device
*APP on Android and IOS
*Build-in user platform
List of the accessories:
Object Quantity
Host 1
Therapeutic handle 1
Therapeutic head Diameter of 6/15/25mm each 1
Power line 1
Silicone cap 10
Aluminum alloy suitcase 1
Product manual 1
Package information:
*Dimension: 300*240*180mm(L/W/H, the device)
*Weight: 6Kg (the device)
*Package method: carton, there is also a aluminum alloy case package inside
Warranty:
3 years for the controller unit, 1 year for the accessories.
Clinical effect:
*Body shapingAnti-cellulite treatment
*Scar and wrinkle smoothing
*Skin elasticity improvement
*Connective tissue tightening
*Treatment of pregnancy stretch marks
*Treatment of post-liposuction irregularities
Applications:
*Physiotherapy
*Rehabilitation
*Sports medicine
*Orthopedics
*Aesthetic medicine
*Treatment for urological pain therapy and erectile dysfunction
Indications:
1,Physiotherapy
*radial and ulnar epicondylitis
*tendinitis of the shoulder/shoulder problems
*calcific tendinitis of the shoulder
*acromion pain
*status post muscular injury
*patellar tendinitis
*patellar tendinopathy
*achillodynia/Achilles Tendonitis
*plantar fasciitis
*heel spurs
*myofascial trigger point therapy e.g. neck
*myofascial trigger point therapy e.g. back, muscular back pain
*trochanteric bursitisperiostitis/shin splints(condition after overload)
*dupuytren's disease
*thumb basal joint arthritis/rhizarthritis
*metatarsalgia
2,Aesthetic for cellulite
*Soft cellulite, stage 1
*Medium cellulite, stage 2
*Hard cellulite, stage 3
*connective tissue/skin tightening
*stretch marks
*After Cryolipolysis treatment
*After radiofrequency treatment
*After ultrasonic treatment
3,ED treatment
*Erectile Dysfunction
Demo vedio of the shockwave therapy device
https://*/watch?v=xu7Tbmr9dl4
Certificates:
CE FDA ISO13485

|
SUPPLIER PROFILE
|
|||
|---|---|---|---|
| Company: | Shenzhen Oceanus Medical Device Co., Ltd | ||
| City/State | Shenzhen, Guangdong | Country: |
China 
|
| Business Type: | Export - Manufacturer / Trading Company | Established: | 2014 |
| Member Since: | 2019 | Contact Person | Julie Wu |
SUPPLIER PROFILE
City/State/Country -
Shenzhen, Guangdong
China 

Business Type -
Export - Manufacturer / Trading Company
Established -
2014
Member Since -
2019
Contact Person -
Julie Wu



