Advertisements
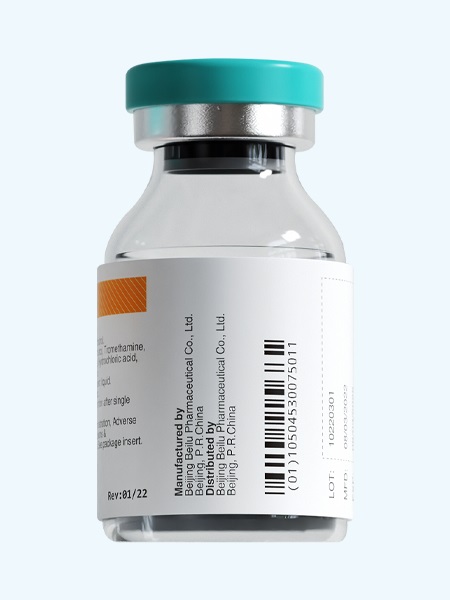

Gadobutrol Injection/API
| Price: | € 10 / Acre |
|---|---|
| Minimum Order: | |
| Payment Terms: | TT |
| Port of Export: |
Product Details
| Model No.: | Brand Name: |
|---|
| Certification: | |
|---|---|
| Specification: | Product name: Gadobutrol Injection |
Packaging & Delivery
| Packaging: | |
|---|---|
| Delivery/Lead Time: | |
| Production Capacity: |
Product Description
In 2020, Beilu launched its macrocyclic Contrast Media- Gadobutrol Injection, it is gadolinium-based non-ionic Contrast Media, a paramagnetic Contrast Media used in various parts of the body lesion (including the brain and spinal cord) MRI imaging and angiography examination. Its macrocyclic nature ensures it is well-tolerated with a good safety profile for MRI Contrast. As a professional contrast media manufacturer, Beilu can provide you with the high concentration gadobutrol gadovist that leads to a better image with lower volume. We also provide DTPA acid with high quality.
Product name: Gadobutrol Injection
Generic name: Gadobutrol Injection
Type: Injectable Contrast Media for MRI, Macrocyclic Contrast Media
Ingredients: Gadobutrol
Specification:gadovist 7.5 ml: 4.5354 g
Administration: Intravenous administration only.
Adverse reactions: See the details from the package insert.
Shelf life: 24 months
Indication of Gadopentetate Dimegumine Injection
Magnetic resonance imaging of the central nervous system (brain and spinal cord), abdomen, chest, pelvis, limbs and other human organs and tissues.
Precautions of Gadopentetate Dimegumine Injection
1. Use with caution in patients with severe kidney damage, epilepsy, hypotension, asthma and other allergic respiratory diseases and those with allergic tendencies.
2. Pay attention to avoid the extravasation of the liquid during the gadobutrol injection uses so as to prevent tissue pain.
3. The serum iron and bilirubin levels of some patients will increase slightly after medication, but they are asymptomatic and can return to normal within 24 hours.
4. Pregnant women and breastfeeding women should use it with caution. Animal experiments show that a small amount of medicine liquid enters the milk.
5. The effective enhancement time of this product is 45 minutes. MRI examination should be performed immediately after intravenous injection.
6. The remaining medicine liquid after one examination should not be used again.
7. When applying the gadovist dose, follow the relevant safety regulations in magnetic resonance imaging.
8. GBCAs should be used with caution. When plain scan MRI cannot obtain the corresponding vital diagnostic information, GBCAs can be used, and the lowest approved dose is used as much as possible.
9. Gadolinium deposition
Current evidence shows that after repeated use of GBCAs, trace amounts of gadolinium can remain in the brain and other body tissues. Research reports have shown that multiple uses of GBCAs can increase the intensity of brain signals, especially in the dentate nucleus and globus pallidus. Currently, there are more reports about linear GBCAs and fewer reports about macrocyclic GBCAs. Animal experiments have shown that the amount of gadolinium deposited after repeated use of linear GBCAs is higher than that of repeated use of macrocyclic.
The clinical significance of brain gadolinium deposition is unclear.
In order to minimize the potential risks associated with gadolinium deposition in the brain, it must be used in strict accordance with the indications and approved doses. It is recommended to use the lowest approved dose that meets the requirement of diagnosis and perform a careful benefit-risk assessment and patient informed communication before repeated administration.

|
SUPPLIER PROFILE
|
|||
|---|---|---|---|
| Company: | Beijing Beilu Pharmaceutical Co., Ltd | ||
| City/State | Beijing, | Country: |
China 
|
| Business Type: | Export - Manufacturer / Trading Company | Established: | 1992 |
| Member Since: | 2022 | Contact Person | beilu pharma |
SUPPLIER PROFILE
City/State/Country -
Beijing,
China 

Business Type -
Export - Manufacturer / Trading Company
Established -
1992
Member Since -
2022
Contact Person -
beilu pharma



