Advertisements
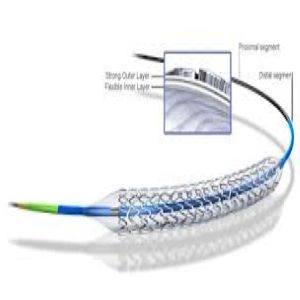

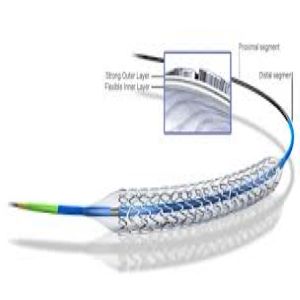
Boston Scientific Promus Element Plus Stent
| Price: | US$ 200 / Pack |
|---|---|
| Minimum Order: | 2 Packs |
| Payment Terms: | L/C, T/T, D/P, D/A, O/A |
| Port of Export: | Tughlaqabadh |
Product Details
| Model No.: | Promus Element Plus | Brand Name: | Boston Scientific |
|---|
| Certification: | FDA, ISO, BSI |
|---|---|
| Specification: |
PROMUS Element™ Plus
Everolimus-Eluting Platinum Chromium Stent System |
Packaging & Delivery
| Packaging: | 1 piece per pack |
|---|---|
| Delivery/Lead Time: | 5 |
| Production Capacity: | 1000 Packs |
Product Description
PROMUS Element™ Plus
Everolimus-Eluting Platinum Chromium Stent System
Indications, Safety, and Warnings
CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the complete “Directions for Use” for more information on Indications, Contraindications, Warnings, Precautions, Adverse Events, and Operator’s Instructions.
INDICATIONS FOR USE
The PROMUS Element™ Plus Everolimus-Eluting Platinum Chromium Coronary Stent System is indicated for improving luminal diameter in patients, including those with diabetes mellitus, with symptomatic heart disease or documented silent ischemia due to de novo lesions in native coronary arteries ≥2.25 mm to ≤4.00 mm in diameter in lesions ≤34 mm in length.
CONTRAINDICATIONS
Use of the PROMUS Element Plus Everolimus-Eluting Platinum Chromium Coronary Stent System is contraindicated in patients with known hypersensitivity to: 316L stainless steel or platinum ∙ everolimus or structurally-related compounds ∙ polymers or their individual components, Primer Polymer and Drug Matrix Copolymer Carrier). Coronary Artery Stenting is contraindicated for use in: Patients who cannot receive recommended antiplatelet and/or anticoagulant therapy (see Section 6.2, Pre- and Post-Procedure Antiplatelet Regimen for more information). ∙ Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the stent or delivery device.

|
SUPPLIER PROFILE
|
|||
|---|---|---|---|
| Company: | Lakshay Surgicals | ||
| City/State | New Delhi, Delhi | Country: |
India 
|
| Business Type: | Export - Manufacturer / Trading Company | Established: | 2012 |
| Member Since: | 2019 | Contact Person | ANIL CHARAYA |
SUPPLIER PROFILE
City/State/Country -
New Delhi, Delhi
India 

Business Type -
Export - Manufacturer / Trading Company
Established -
2012
Member Since -
2019
Contact Person -
ANIL CHARAYA



